IODIDE KINETICS with TYROSYL PEPTIDE IODINATING
SYSTEM RECONSTITUTED FROM THE THYROID
PREFACE
Following is a dissertation on the "Resolution and Reconstitution of the NADPH-Dependent Tyrosyl-Peptide Iodinating Activity from Porcine Thyroid Tissue". Analytical techniques unavailable at the time this dissertation was written have since confirmed this mechanism, its' importance to thyroid function, and the probable identity of the unidentified microsomal factor that is necessary for proper thyroid function and regulation.
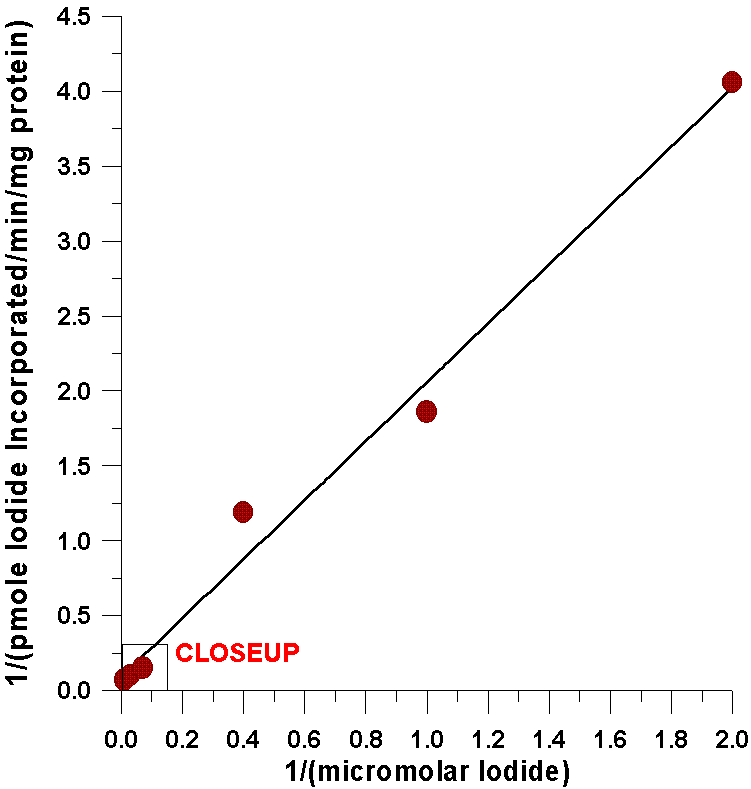
The first evidence comes from kinetic analysis
of the iodide-incorporating system found in the thyroid with respect to
substrate concentrations of iodide. A double reciprocal plot indicates
that the kinetics for iodide are completely linear over a very broad range
of iodide concentrations. This degree of linearity has never been accomplished
with peroxide-dependent iodinating reactions which typically display substrate
inhibition and non-linear iodide kinetics. Combined with the fact that
the activity of thyroid peroxidase is virtually eliminated (99.5%) by physiological
concentrations of glutathione, this information provides strong evidence
that the iodination of tyrosine residues in the thyroid, and perhaps even
the complete biosynthesis of thyroid hormone, occurs through a mechanism
other than that catalyzed by "thyroid peroxidase".
IODIDE KINETICS with TYROSYL PEPTIDE IODINATING
SYSTEM RECONSTITUTED FROM THE THYROID

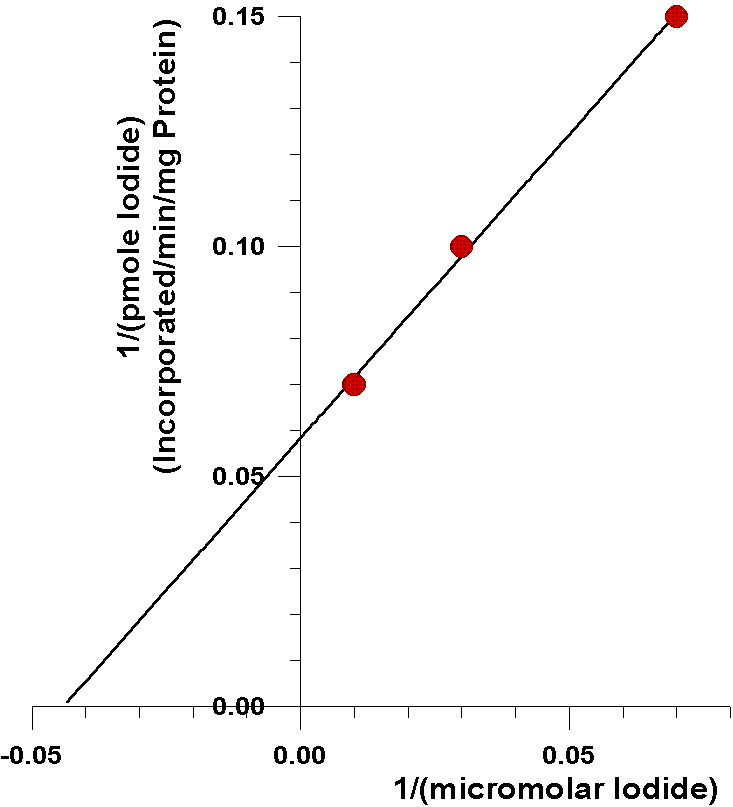
As can be seen from the following closeup of
this data, the amount of iodide half-saturating incorporation of iodide
into tyrosyl residues (23µM) is considerably less than the amount
of iodide half-saturating the oxidation of iodide catalyzed by the flavin-containing
monooxygenase (0.57 mM). There are several possible explanations for this
phenomena. The sulfenic acid of vitaletheine or its' sulfenyl iodide, or
both, may competitively inhibit further oxidation of iodide, thereby helping
to protect NADPH and NADH (both critical to the activities of this monooxygenase)
from irreversible oxidation by iodine (I2). Utilization
of these oxidative intermediates is theoretically more efficient since
the sulfenic acid, essentially a thioperoxide, may be far less toxic to
sensitive biological components than peroxide and less likely to be lost
in side reactions. Thus, monooxygenase-catalyzed reactions in which the
active iodine species are immediately incorporated into tyrosyl residues
are expected to have a lower Km for iodide and other
substrates. In contrast, "peroxide-dependent" iodinations catalyzed by
"thyroid peroxidase" fail to protect these reduced pyridine nucleotides
and many other sensitive biological structures from iodination and oxidation.
Km DETERMINATION FOR IODIDE IN RECONSTITUTED
IODINATING SYSTEM FROM THE THYROID
The proposed sulfenate and sulfenyl iodide mechanism for iodinations occurring in the thyroid is made even more plausible by the finding that vitalethine is bound directly to its enzyme receptor. A peak at 383, exactly the mass ion expected for vitalethine, was observed when highly purified monooxygenase from hog liver was analyzed in a MALDI mass spectrometer. This enzyme has been shown to oxidize other thiol substrates, such as cysteamine, to its' sulfenic acid, which then reacts with unreacted thiol substrate to form the disulfide, cystamine. Water is split off as a by-product of this reaction. Through a similar reaction of the sulfenic acid (VSOH) of vitaletheine (VSH), the disulfide (VSSV), vitalethine, can be formed. Vitaletheine even contains the cysteamine moiety known to be a substrate for the monooxygenase.
2 VSH ====[Oxidation]====> VSH + VSOH ====> VSSV + H2O
A mass ion on this monooxygenase more characteristic of the sulfenyl iodide or sulfenyl periodide may be possible when iodide is abundant or when the enzyme is isolated from thyroid tissue. When vitaletheine is limiting and iodide and other substances are completely absent it might even be possible to detect the mass ion of the sulfenic acid.
RSH + I- ==[Oxidation]==> RSOH + I- =====> RSI + OH-
With a variety of analytical and kinetic techniques this dissertation confirms that an enzyme similar to the hepatic, microsomal FAD-containing monooxygenase is absolutely necessary for any controlled and specific iodinating reactions occuring in thyroid tissue under physiological conditions. Demonstration that the immediate precursor of vitaletheine, 4'-phosphopantetheine, is needed for optimum peroxide-independent iodinating reactions means that uncontrolled "thyroid peroxidase"-catalyzed reactions probably occur only when the thyroid is poisoned or deficient in the very nutrients needed to support the biosynthesis of vitaletheine and its' enzyme receptor, the monooxygenase. As such, the enzyme activity known as "thyroid peroxidase", and widely taught as the enzyme responsible for formation of thyroid hormone for about four decades, is probably an artifact of isolation procedures. An iodotransferase activity is probably more descriptive of the biological activity of this hemoprotein under physiological conditions.
Since thyroid hormone (T4) first must be activated to tri-iodothyronine (T3) before it becomes biologically active, and since T3 is known to cause the genetic expression of a host of regulatory enzymes and proteins, this new information on how the thyroid hormone is formed may not only dramatically improve our understanding of the causes of disease, but might also help us to develop effective nutritional and environmental solutions for serious and intractable health problems. The monooxygenase and vitalethine are again players in this activation process, since the deiodinating reaction from T4 to T3 requires a thiol substrate. The monooxygenase and vitalethine probably control the availability of such thiol substances (RSH) by oxidizing them to disulfides, thereby attenuating and regulating the deiodinating process.
VSH ==[Oxidation]==>
VSOH
+ RSH =====> VSSR + H2O
VSSR + RSH =====> VSH
+ RSSR
| Home | Overview | People | Journal | Nutrition |
| Environment |
|
WWW Links | Outline | e-mail us |